1886 Explanation of the Telegraph Battery - Basic Design | |
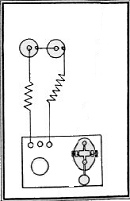
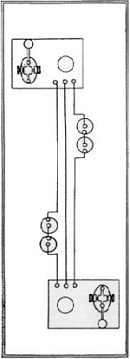
| The telegraph consists in a combination of four parts: 1) a battery, which produces the electrical current The CPR and CNR required all student operators to become thoroughly familiar with all the practical features of the apparatus and mechanisms of the telegraph. It is not only a great aid to the prospects of advancement for the student operator to have a thorough knowledge of the electrical theory of telegraphing and to understand all about the batteries, the wires, and the instruments. THE BATTERY: This is the first and most essential part of a telegraphic apparatus. It is by the chemical action in the battery that the electric current is made to traverse long or short distances through the conducting medium of iron metallic wires, and by means of the proper instruments made to give out tangible signals which, being in the form of an alphabet, enables us to read or speak instantaneously through great distances, for the electric requires but a small fraction of a second's time to travel many hundreds of miles through the wires. The basic battery in 1886 was known as the "Gravity Battery." It consisted of three parts: the jar, the zinc, and the copper. The jar is of glass and is about five inches diameter and seven inches deep. The Zinc is provided with a brass connecting screw at the top of the arm - the arm serves as a means of supporting the zinc in a proper position. | The connecting screw is used to bind or connect a copper wire to the zinc, which is called the "negative" or "zinc pole" of the battery. When the battery is charged for operation if the wire projecting upward from the copper be connected with the zinc by binding the bared end of the wire from copper to zinc, a current of electricity will constantly flow through the wire from copper to zinc, and will cease to flow the moment the wire is disconnected. If the wire from the copper be extended to a mile in length, and its end connected in the same manner with the zinc, the current will flow through the entire length and come back to the zinc, just as surely as though the distance were but a few inches, and will instantaneously cease to flow the moment the wire is disconnected or broken at any point in its entire length. Where powerful currents are required, additional cells are added by connecting either the copper or zinc pole of the first cell to the opposite pole of the next, and so on; so that in a series of fifteen or twenty cells, if the unconnected pole of the cell at the one end was copper that pole would constitute the copper pole of the entire battery, and the unconnected zinc at the other end would be the zinc pole of the entire battery. By connecting the end of the wire of any length to the zinc or copper pole of such a battery, and its opposite end to the remaining pole, a much more powerful current would pass through the wire than if the battery consisted of but one cell. Telegraph companies on their long lines use batteries of from twenty to one hundred cells each. See some examples below: |
 |  |  |  |  |  |